Abstract
Background: Persistence of minimal residual disease (MRD) is associated with a high rate of treatment failure, relapse, and death in B-cell acute lymphoblastic leukemia (B-ALL). Particularly, patients who remain MRD positive before an allogeneic hematopoietic stem cell transplant (HSCT) have an expected disease-related death rate due to the disease of about 60%. The T cell-engager CD19-directed therapy blinatumomab demonstrated considerable efficacy in this setting, however resistant or unsuitable patients have no alternative therapy. Inotuzumab Ozogamicin (IO) is a CD22-directed antibody-drug conjugate with remarkable efficacy in B-ALL with overt disease. Furthermore, IO does not need a high effective immune system to exert activity.
Methods: In this multicenter phase 2 trial (NCT03610438), we are investigating the efficacy of IO in obtaining MRD negativity in two cohorts of 38 Ph+ and 38 Ph- B-ALL patients. IO was administered at the dose of 0.5 mg/sqm on days 1, 8, and 15 of a 28-days cycle. Patients who did not obtain MRD negativity after the 1st IO cycle had the option to receive the 2nd IO cycle maintaining the same dosage. Responding Ph- patients received short-term maintenance with low-dose chemotherapy (alternating vincristine, cyclophosphamide, prednisone, methotrexate, and 6-mercaptopurine), responding PH+ patients received tyrosine kinase inhibitor (TKI) for up to 12 weeks, allowing proper IO washout before HSCT. HSCT ineligible patients entered into low-dose chemotherapy or TKI maintenance for up to 2 years. For the primary endpoint, MRD was centrally assessed at baseline, after course 1, after course 2, and eventually before transplant with high sensitive molecular methods for BCR-ABL1 or V(d)J fusion transcripts. Here, we present the results of an unplanned interim analysis that was generated to evaluate the continuation of the study considering the slow accrual due to the Sars-COV2 pandemic.
Results: To date, 51 patients underwent screening procedures, and 43 were considered eligible. We report data from the first 39 patients treated in the study. Nineteen out of 39 patients had Ph- B-ALL and 20/39 Ph+ B-ALL. Eighteen out of 39 (46%) patients were male. The median age was 55 years (22-84), 15/39 patients (38%) were older than 65 years. Demographics were comparable between Ph+ and Ph- patients. Within Ph+, ALL 39% of patients harbored p190 and 61% p210 fusion transcript. Within Ph- B-ALL, 1 patient harbored ALL1/AF4 and 1 patient harbored ALL1/ENL fusion transcript. Most of the patients received 1 (16/39, 42%) or 2 (19/39, 50%) previous lines of therapy; 5 patients (13%) were resistant to blinatumomab, and all of them had Ph- B-ALL.
Twelve out of 19 (63%) Ph- B-ALL patients received only 1 IO cycle, 7/19 (37%) received 2 cycles; 5/20(25%) Ph+ B-ALL received only 1 IO cycle, and 15/20 (75%) received 2 cycles. To date, central MRD monitoring is available for 20 patients (12 Ph- and 8 Ph+ B-ALL, ongoing for the remaining cases). Overall, MRD negativity was obtained in 7/20 patients (35%); Particularly, in Ph- B-ALL cohort 5/12 (42%) patients achieved MRD negativity, 4/12 (38%) achieved disease reduction at low level MRD positivity (<10-4); in Ph+ B-ALL cohort, 2/8 (25%) patients achieved MRD negativity. One out of 5 (20%) blinatumomab pre-treated patients obtained MRD negative response.
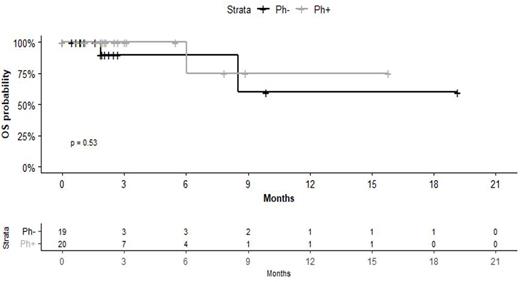
IO was an effective bridge to HSCT for 10/39 patients (26%; in patients <65 years old, 9/24 patients, 37%); Specifically, all 7 patients who obtained MRD negativity after IO, a patient with low-positive MRD and 2 patients with non yet available MRD data were bridged to HSCT. Accordingly, with the caveat of a short median follow-up (2.8 months, range 0.6-20.0 months), overall survival in the study population seems promising (figure 1, median OS not reached).
Overall, adverse events collected during the study were limited in number and grading. Also, myelotoxicity was manageable. Out of 39 patients, we documented 1 veno-occlusive disease (2.5%) that occurred within 20 days from the last IO dose and before any HSCT.
Conclusions: IO administration demonstrated activity with a good safety profile in the setting of MRD positive B-ALL, including blinatumomab resistant patients. Importantly, IO was capable to bridge 37% of eligible patients to HSCT, augmenting the chance to achieve a cure. The continuation of the study will deliver mature efficacy and survival data for a proper evaluation of this promising strategy.
Disclosures
Marconi:pfizer: Honoraria, Research Funding, Speakers Bureau; servier: Honoraria; astellas: Honoraria; menarini/stemline: Honoraria, Speakers Bureau; abbvie: Research Funding. Chiaretti:Amgen: Other: advisory board; Incyte: Other: advisory board; Abbvie: Other: advisory board; Gilead: Other: advisory board; Pfizer: Other: advisory board. Papayannidis:Abbvie: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Blueprint: Honoraria; Incyte: Honoraria; GlaxoSmithKline: Honoraria; Bristol Myers Squibb: Honoraria. Rambaldi:Janssen: Honoraria; Roche: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Celgene-BMS: Honoraria; Omeros: Honoraria. Fracchiolla:Amgen: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Jazz: Honoraria, Speakers Bureau; Pfizer: Research Funding, Speakers Bureau. Candoni:Celgene, Abbvie, Pfizer, Janssen, Astellas, Jazz, Gilead, Incyte, Amgen: Other: Speaker honoraria. Bochicchio:werfen, Imstrumentation Laboratory: Consultancy, Honoraria. Vignetti:Astrazeneca: Speakers Bureau; AbbVie: Speakers Bureau. Martinelli:Astellas: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Abbvie: Consultancy; Roche: Consultancy; Daiichi Sankyo: Consultancy; Stemline: Consultancy; Incyte: Consultancy; Celgene/BMS: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy.
OffLabel Disclosure:
Inotuzumab is used for MRD positive ALL instead that in patient with overt disease
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal